
zip file containing this book to use offline, simply click here. When pKa is close to pH, there is an ideal balance between salt and acidity, maximizing effectiveness of the acid. You can browse or download additional books there. More information is available on this project's attribution page.įor more information on the source of this book, or why it is available for free, please see the project's home page. Because it uses small decimal quantities to explain acid dissociation, pKa is used. Lactic acid is a more vital acid than acetic acid, as evidenced by its pKa values. Acetic acid, for example, has a pKa of 4.8, and lactic acid has a pKa of 3.8. Additionally, per the publisher's request, their name has been removed in some passages. The acid is more potent if its pKa value is lower. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Normally, the author and publisher would be credited here. The negative log of the acid dissociation constant, or Ka value, is pKa. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. The pKa value is one way for determining an acids strength. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP).
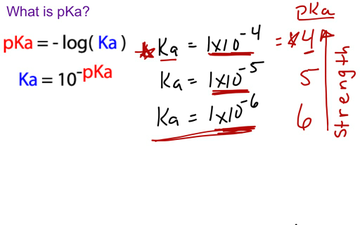
This book is licensed under a Creative Commons by-nc-sa 3.0 license.


 0 kommentar(er)
0 kommentar(er)
